MinerAlert

MinerAlert
After IRB approval, any changes to the study must be reviewed and approved by the IRB prior to implementation. This includes changes to the research team, recruitment process or material, consent procedures or materials, participant payment, data collection procedures, and target population. Protocol violations are changes in the approved IRB protocol that are under the investigator's control and made without prior IRB approval.
When changes to the IRB are necessary to immediately eliminate or reduce an apparent hazard to the safety of research participants or others, those changes may be initiated without prior IRB approval. This event must be reported to the IRB within 5 working days of initiation. If there are subject safety concerns, contact the IRB Administrator immediately.
Modifications are made and submitted in IRBNet. You will need to create a new amendment package. They must also edit appropriate sections of the IRB and attach revised documents.
All Documents may be found on our forms page
Common Documents Submitted
A protocol deviation is any change, deviation, or departure from the approved IRB that is under the researcher's control and has not been approved by the IRB. Upon discovery, the Principal Investigator and research team is responsible for reporting protocol deviations to the IRB.
An event that does not impact subject safety, compromise the integrity of study data and/or affect a subject's willingness to participate in the study. Minor deviations or violations will be reviewed by the IRB Chair. The IRB Chair will determine whether the event is accepted as a minor deviation/violation and can recommend a corrective course of action.
An event that may impact subject safety, compromise the integrity of study data and/or affect a subject's willingness to participate in the study. Major protocol deviations/violations are treated as noncompliance and will be reviewed by the IRB to determine the appropriate course of action.
Incidents are submitted in IRBNet. Additional information may be requested by the IRB after submitting the Incident.
An IRB shall have authority to suspend or terminate approval of research that is not being conducted in accordance with the IRB's requirements or that has been associated with unexpected serious harm to participants. Any suspension or termination of approval shall include a statement of the reasons for the IRB's action and shall be reported promptly to the researcher and appropriate institutional officials or the department or agency head.
All Documents may be found on our forms page
Common Documents Submitted
Researchers may encounter certain types of unanticipated problems, adverse events or protocol deviations. Researchers must report these incidents to the IRB.
An incident or event that was unexpected by the research team. This incident or event has developed a new or increased risk to the participants and was possibly related to the research procedures.
Unpleasant or unfavorable medical occurrence in a participant, including any abnormal sign, symptom, or disease, temporally associated with the research participation. Adverse events encompass both physical and psychological harms.
All Documents may be found on our forms page
Common Documents Submitted
Exempt reviews do not require continuing review by the IRB, but do require project status reports.
Occasionally, the Board may determine that the project risk warrants a continuing review for expedited projects. However, Most expedited projects only require project status reports.
UTEP IRB requires that exempt and expedited projects not requiring continuing review submit a project status report every 2 years. To facilitate this, projects are issued an expiration date that is 2 years from the initial decision date. PIs should submit a project status report to inform the IRB that the project is continuing. PIs may also include any amendments that may be needed in the project status report.
Projects that do not require continuing review may be administratively closed if a project status report is not received at the issued expiration date. At that point, all research activities must stop immediately until a project status report is approved.
All Documents may be found on our forms page
Common Documents Submitted
Full Board projects require continuing review. The maximum allowable interval between reviews is 1 year. The IRB may determine that continuing review needs to be more frequent based on the risk of the project. Projects that require continuing review expire when continuing review is due.
PIs are notified by email starting three months prior to when their project is set to expire. Expired projects are in non-compliance and all research activities must stop immediately until a continuing review is approved.
All previously approved, or approved as amended, project documents must be submitted as part of the continuing review. Any revisions or changes to previously approved documents that are introduced as part of the continuing review must be made on the most current version of the document and accompanied by an amendment form. The convened IRB will review all documents and make an appropriate determination.
All Documents may be found on our forms page
If all research interventions or interactions with participants have been completed and collection and analysis of identifiable private data/biospecimens are finished, the study should be closed with the IRB.
Once a study has been closed, investigators may keep the data collected (including identifiable, private data) if consistent with the IRB-approved protocol.
Investigators should continue to honor any confidentiality protections of the data. Investigators should also honor any other commitments that were agreed upon as part of the approved research. For example, providing information about the study results to research participants or honoring reimbursement commitments for participation. IRBs that remain active are subject to audit by the IRB.
All Documents may be found on our forms page
Common Documents Submitted
Create a new package
Click on the title of your project in "My Projects". Then click on "Create a New Package".

Go to Designer
Attach all study documents. Please use the current versions available for download in Designer.


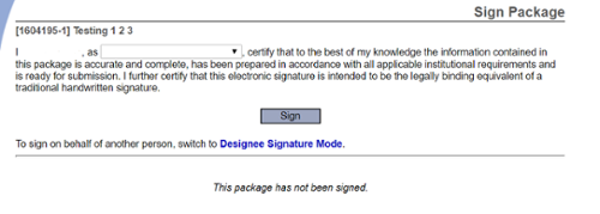
Sign this Package
Sign the package, be sure to choose the role that best describes your role in the research project, e.g. Principal Investigator, Research Coordinator, etc.

Submit this Package
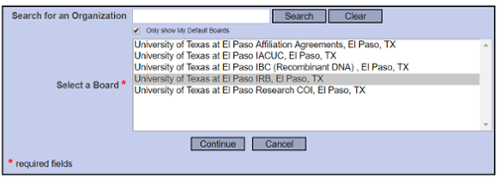
Select “University of Texas at El Paso IRB, El Paso, TX” and continue to submit.